This question has caused many sleepless nights and initiated countless discussions within the industrial and even transportation sectors. Before examining the causes for lubrication failure, one must first consider the definition of lubricant failure.
The composition of a liquid lubricant can be described as a combination of base oil and additives (Menezes, Reeves and Lovell 2013, 295). These two components work in tandem to define particular characteristics of the lubricant to perform its functions. According to Menezes, Reeves and Lovell (2013, 296) the five functions of a lubricant include; the reduction of friction, minimization of wear, distribution of heat, removal of contaminants and improvement of efficiency. As such, lubrication failure can then be described as the failure of a lubricant to adequately perform any or a combination of its five functions as a result of the degradation of any of its two components; namely the base oil or additive package. Thus, it can be deduced that lubrication failure is as a result of lubricant degradation.
Now that we understand that a lubricant fails when it undergoes degradation which by extension results in the lubricant not being able to perform any of its functions properly, we need to explore further on the types of degradation that exist. Only then can we really answer the question of how a lubricant can fail.
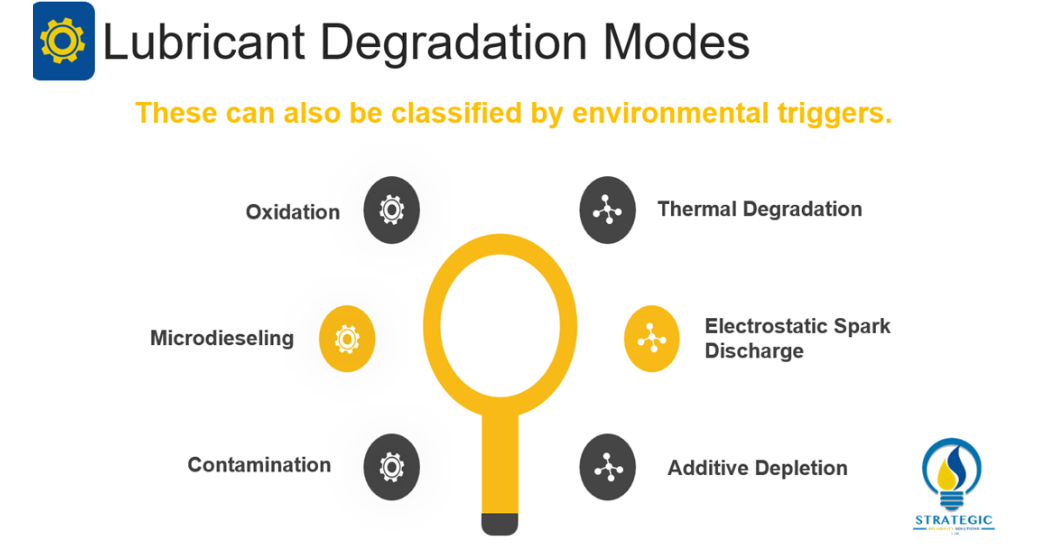
Barnes (2003, 1536) focuses on three main mechanisms of lubricant degradation namely; Thermal Degradation, Oxidation and Compressive Heating (Microdieseling) [2]. One may argue that these three types form the basis of all mechanisms of lubricant degradation however, Livingstone, Wooton and Thompson (2007, 36) identify six main mechanisms of degradation namely; Oxidation, Thermal Breakdown, Microdieseling, Additive Depletion, Electrostatic Spark Discharge and Contamination [1]. In this instance, the six identified mechanisms all produce varying identifiable characteristics which lend to these six forming the foundation of identification of lubricant degradation mechanisms.
With these six in mind, one would need to be able to determine which degradation mechanism is at work in their facility. Afterwards, methods to treat with these mechanisms must be administered. Let’s understand each mechanism.
Oxidation
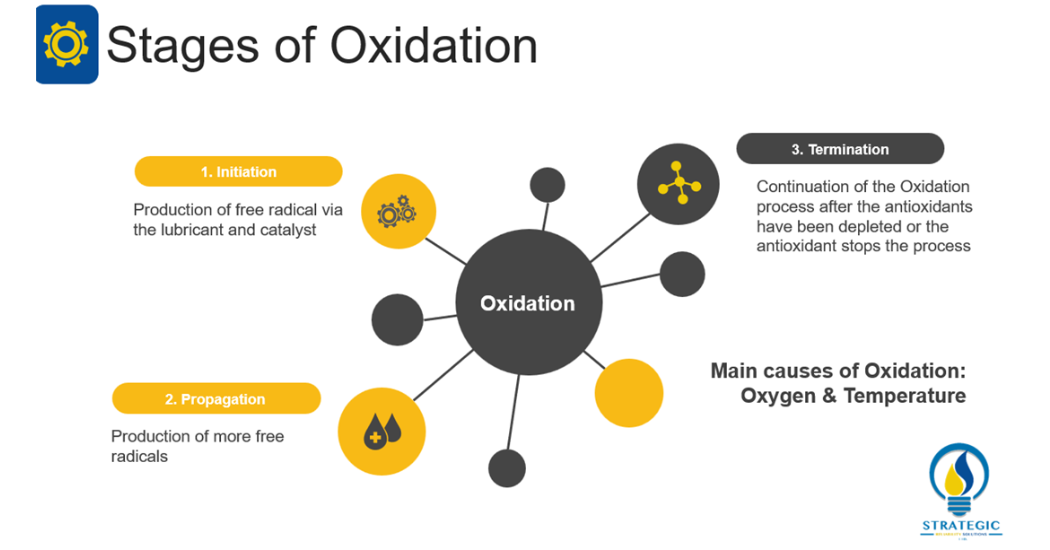
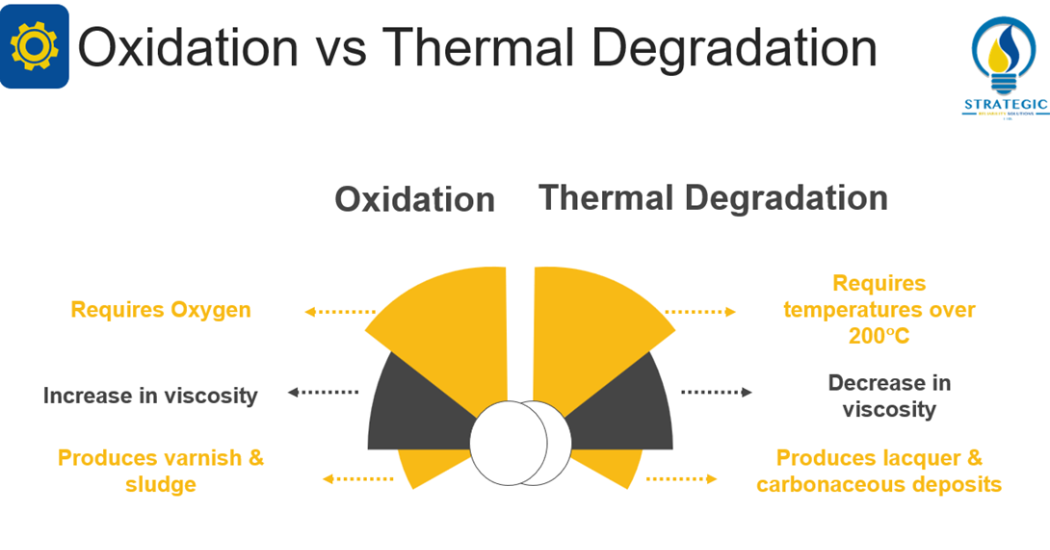
This mechanism involves the reaction of oxygen with the lubricant. According to Livingstone, Wooton and Thompson (2007, 36) oxidation can result in the formation of varnish, sludge, increase in viscosity, base oil breakdown, additive depletion and loss in antifoaming properties of the lubricant[1]. Barnes (2003, 1536) refers to this phenomenon as the addition of oxygen to the base oil to form aldehydes, ketones, hydroperoxides and carboxylic acids[2]. On the other hand, Wooton (2007, 32) explains that there are three main stages of oxidation namely initiation, propagation and termination[7]. Fitch (2015, 41) explains that initiation entails the production of a free radical via the lubricant and a catalyst[8]. Propagation involves the production of more free radicals via additional reactions. Finally, termination entails either the continuation of the oxidation process after the antioxidants have been depleted or the antioxidant stopping the oxidation process.
Thermal Breakdown
This mechanism is largely dependent on temperature as one of its contributory factors even though dissipation of heat was highlighted above as one of the functions of a lubricant. However, during the operation of machinery particular components tend to develop increasing temperatures. As described by Livingstone, Wooton and Thompson (2007, 36) once this temperature exceeds the thermal stability point of a lubricant, the consequences can include shearing of the molecules [1]. This phenomenon is also called the thermal cracking of the lubricant which can result in the production of unwanted by products, polymerization and decrease in viscosity.
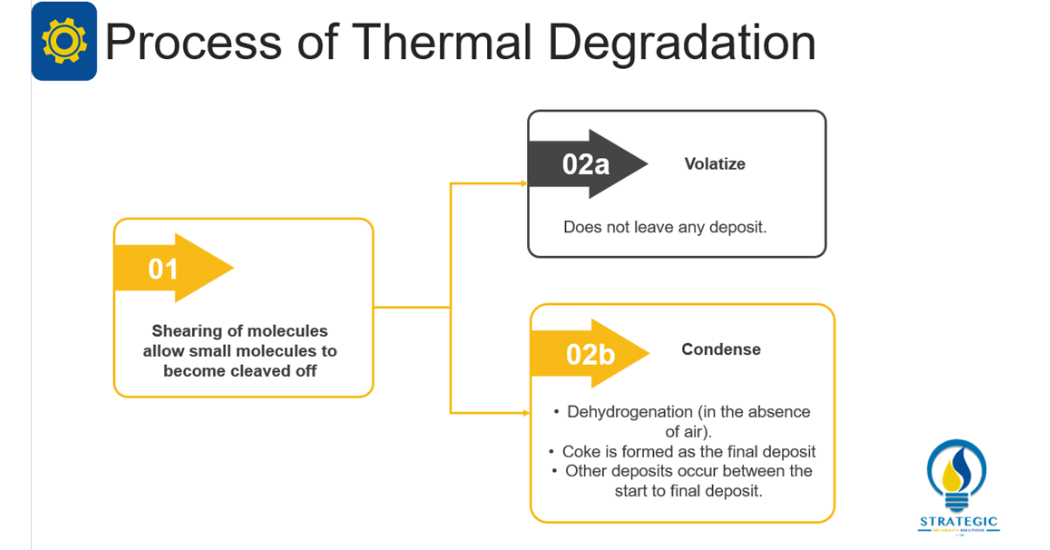
Subsequently, Barnes (2003, 1536) explains that thermal degradation usually occurs when the lubricant experiences temperatures in excess of 200°C [2]. He also states that the by-products of thermal degradation differ from that of oxidation. Wooton and Livingstone (2013) state that there are two main actions that can occur once a lubricant is thermally degraded [4]. Either the small molecules will become cleaved off and volatize from the lubricant. This does not leave any deposit in the lubricant. On the other hand, there is the condensation of the remainder of the molecule in the absence of air thus dehydrogenation also occurs. Consequently, coke is formed as the final deposit with numerous types of deposits forming between the start of the condensation to its final deposit of coke. The main contributing factor for thermal degradation can therefore be linked to dramatic increases in temperature or constant high temperatures.
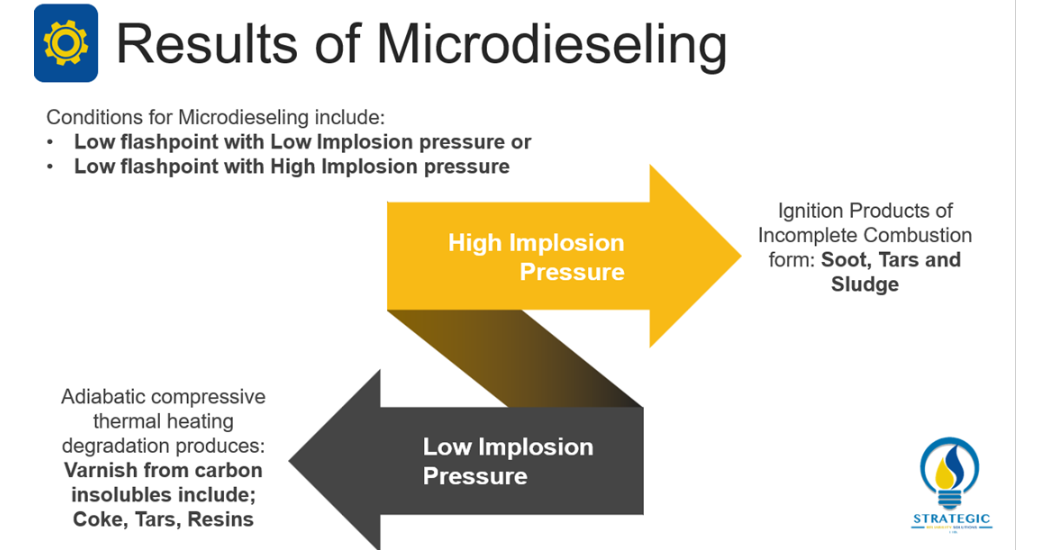
Microdieseling
Livingstone, Wooton and Thompson (2007, 36) have characterized Microdieseling as a form of pressure induced thermal degradation [1]. They describe it as the transition of entrained air from a low pressure to a high pressure zone which results in the adiabatic compression. This type of compression results in localized temperatures almost on excess of 1000°C. As such, the lubricant undergoes dramatic degradation. Wright (2012, 14) explains that because of these high temperatures, the bubble interface becomes carbonized [6]. As such, carbon by products are produced and the oil undergoes oxidation.
Additive Depletion
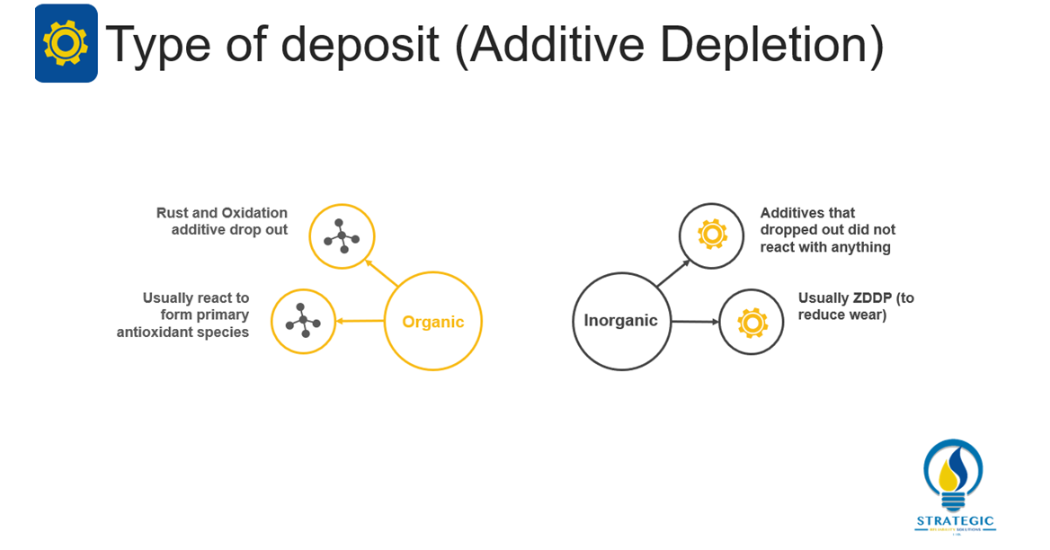
Wooton and Livingstone (2013) indicate that additive depletion can result in either organic or inorganic deposits[4]. The nature of the deposit is dependent on the type of additive that has been depleted and its reaction with other components in the oil. For instance, if the rust and oxidation additives drop out of the oil, they typically react to form primary antioxidant species thus producing organic deposits. However, as Wooton and Livingstone (2013) explain, inorganic deposits can also be formed from additives that have dropped out of the oil but did not react with anything[4]. This unresponsive reaction is typical of ZDDP (Zinc dithiophosphate) which is an additive that assists with reducing wear in the lubricant. In cases of additive depletion, the FTIR test seeks to identify spectra relating to the reacted or unreacted additive packages for the lubricant in use (Wooton and Livingstone, 2013).[4]
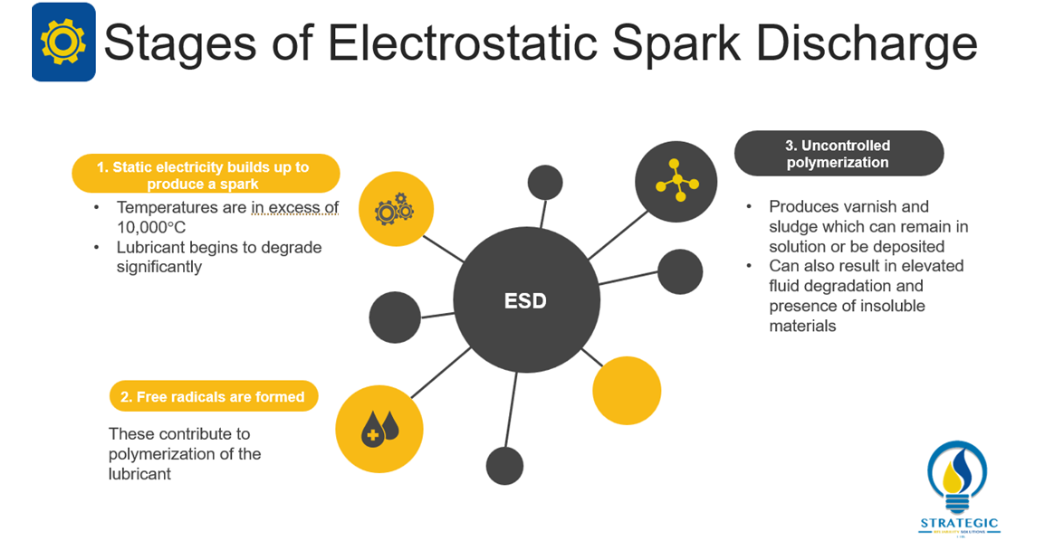
Livingstone, Wooton and Thompson (2007, 36) describe this phenomenon as the generation of static electricity at a molecular level when dry oil passes through tight clearances[1]. It is believed that the static electricity can build up to a point whereby it produces a spark. This spark can induce localized temperatures in excess of 10,000°C which can significantly degrade the lubricant at an accelerated rate. Van Rensselar (2016, 30) also advocates that Electrostatic Discharge contributes to the formation of free radicals in the lubricant which subsequently results in uncontrolled polymerization[5]. This polymerization of the lubricant gives rise to the formation of varnish and sludge which may deposit on the surface of the equipment or remain in solution. Van Rensselar (2016, 32) indicates that the most common result of Electrostatic Discharge is an elevated rate of fluid degradation and the presence of insoluble materials.[5]
Contamination
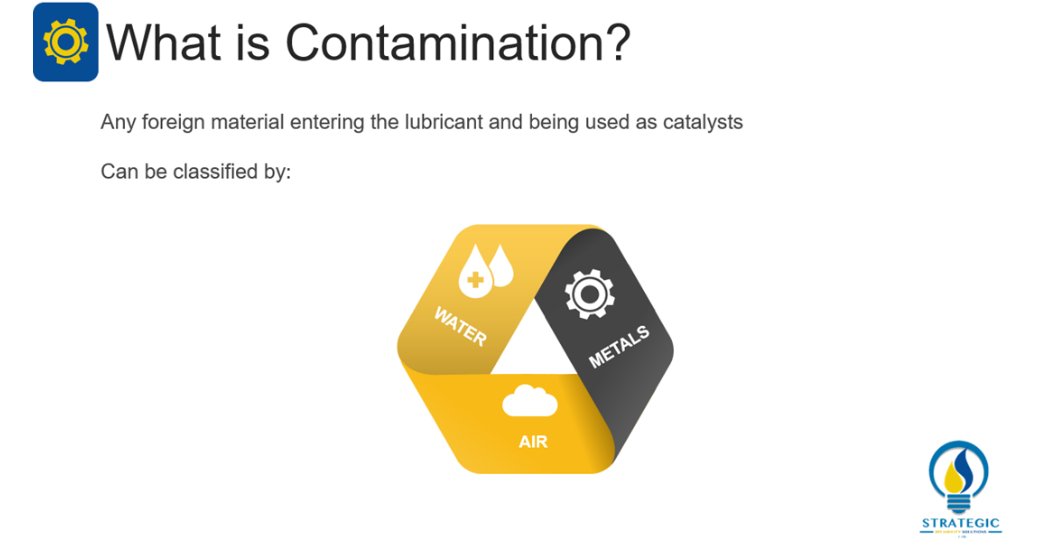
This mechanism of degradation can include foreign material entering the lubricant and being used as catalysts for degradation mechanisms listed above. Contaminants can include a variety of foreign material, however Livingstone, Wooton and Thompson (2007, 36) have narrowed the list to metals, water and air[1]. These main contaminants can significantly contribute to the degradation of the lubricant by oxidation, thermal degradation or compressive heating.
From the above, we can summarize these lubricant degradation mechanisms into the following table:
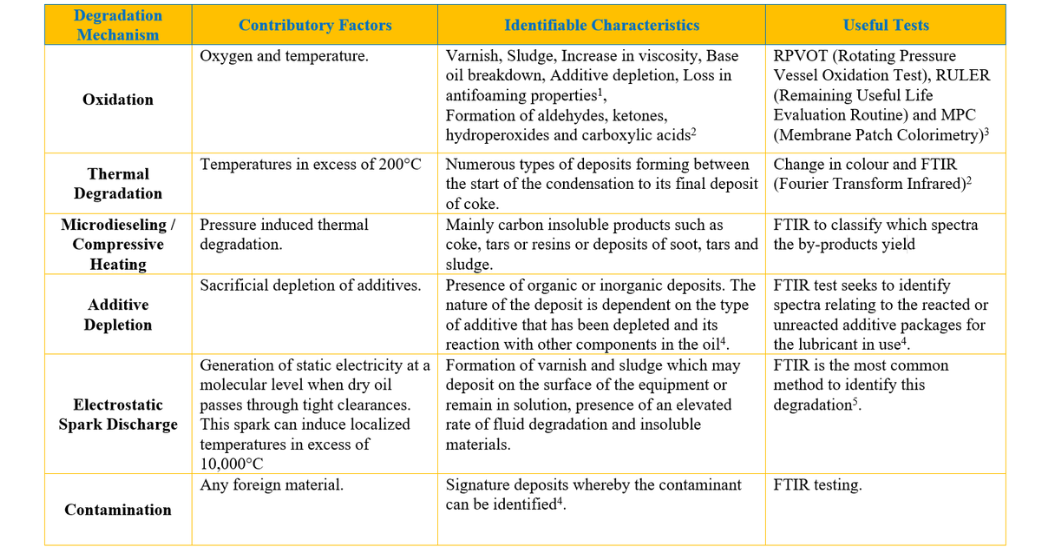 From this summary, we can now assess the methods in which a lubricant can fail. While this article may serve as a guide in determining various lubricant degradation mechanisms, each mechanism must be treated differently depending on the conditions (environmental and operational) that exist during the lubricant failure. A proper root cause analysis should always be done when investigating any type of failure.
From this summary, we can now assess the methods in which a lubricant can fail. While this article may serve as a guide in determining various lubricant degradation mechanisms, each mechanism must be treated differently depending on the conditions (environmental and operational) that exist during the lubricant failure. A proper root cause analysis should always be done when investigating any type of failure.
References
[1] Livingstone, Greg, Dave Wooton, and Brian Thompson. 2007. “Finding the Root Causes of Oil Degradation.” Practicing Oil Analysis, Jan – Feb.
[2] Barnes, M. 2003. “The Lowdown on Oil Breakdown.” Practicing Oil Analysis Magazine, May-June.
[3] Livingstone, Greg and David Oakton. 2010. “The Emerging Problem of Lubricant Varnish.” Maintenance & Asset Management, Jul/Aug.
[4] Wooton, Dave and Greg Livingstone. 2013. “Lubricant Deposit Characterization.” Paper presented at OilDoc Conference and Exhibition Lubricants Maintenance Tribology, OilDoc Academy, Brannenburg, Rosenheim, Germany, United Kingdom, January 22-24, 2013.
[5] Van Rensselar, Jeanna. 2016. “The unvarnished truth about varnish”. Tribology & Lubrication Technology, November 11.
[6] Wright, Jeremy. 2012. “Microdieseling and Its Effects on Oil.” Machinery Lubrication, December.
[7] Wooton, Dave. 2007. “The Lubricant’s Nemesis – Oxidation.” Practicing Oil Analysis Magazine, March.
[8] Fitch, Bennett. 2015. “Identifying the stages of oxidation.” Machinery Lubrication Magazine, June.