The Food and Drug administration (FDA) comprises of a very broad and complex area of regulations. While there is no specific required certificate for FDA approval, it is up to the manufacturer to read and review the regulations to ensure their material/product meet that particular criteria.
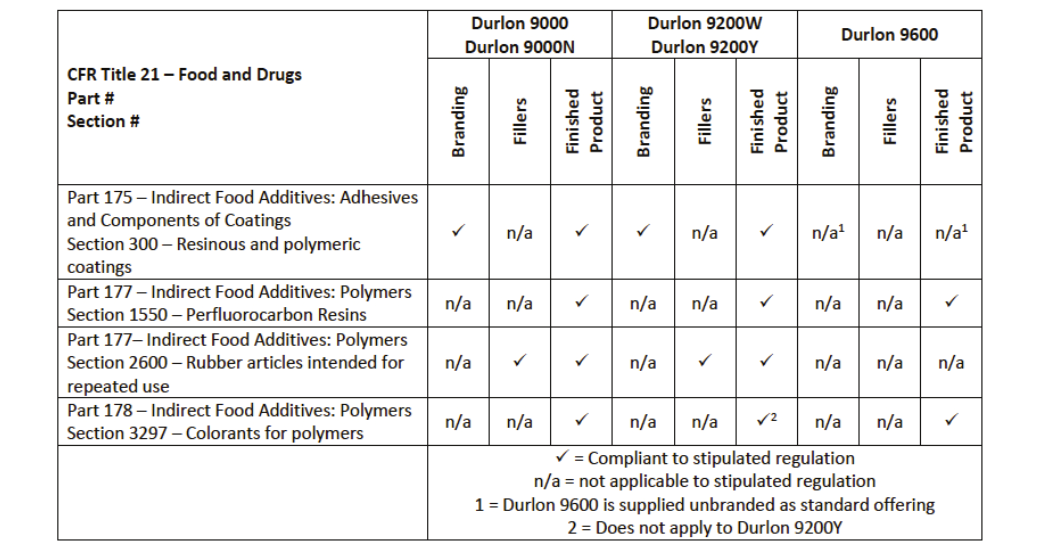
There are multiple ways a gasket can conform to FDA regulations:
- GRAS (Generally Recognized As Safe) – Materials adequately shown to be safe under the conditions of its intended use, or unless the use of the substance is otherwise excepted from the definition of a food additive. Eg. PTFE or 316SS.
- Existing FDA Regulations – Material ingredients that are listed as compliant in the applicable FDA regulations.
- FDA FCN (Food Control Notification)– These are new materials that are not covered by either of the above but can be submitted to the FDA under a FCN.
PTFE and Virgin PTFE gaskets are commonly used in FDA applications. Filled PTFE gaskets can conform to FDA regulations if the filler or fillers, colouring agents, etc., are considered as GRAS or if the fillers and colouring agents are complying with another regulation. If PSA (Pressure Sensitive Adhesive) is used, it will also need to conform to FDA regulations.
Elastomer gaskets are another gasket material that will conform to FDA regulations (For eg. Durlon® 8500). This gasket material is commonly used in both raised-face and flat-faced flanges (in which the available compressive load is low).
If any adhesives are used to perform a sealing function, they will need to meet the requirements mandated by the FDA (21 CFR 175.105).
When inks are used to mark gasket material, it must follow the same FDA (21 CFR 175.105) criteria and all ingredients used in the branding ink must be acceptable for direct contact with food and pharmaceutical products.
Some applications are more complex/difficult and require the use of systems that are over and above the FDA regulations, such as the National Sanitary Foundation (NSF), U.S. Pharmacopeias (USP) and 3A.
It is always important to contact your gasket manufacturer to confirm your gasket conforms to FDA regulations.




