Developments in the Biopharmaceutical Market: Biosimilars
A major development of this decade has been the number of patent expiries of branded drugs. This has given way to a new market of generic biological drugs called “biosimilars.” Branded biological drugs worth more than $80 billion in global sales will lose their patents through 2015, presenting a major opportunity for the metering pumps market in pharmaceutical manufacturing.
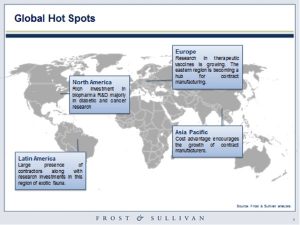
The North American and European regions see large investments in the research and development as manufacturing is largely shifting to regions with cost advantages. Most of the generic drug growth is in the Asia-Pacific (APAC) region as a result of the rising number of contract manufacturers. The price competitiveness of generic drugs has brought a complete change to the industry. The 12th five-year plan of China shows a significant investment in the development of these high value products. Similarly, the Indian biosimilar market also shows an impressive growth rate. Other regions like Eastern Europe and Latin America also show a rise in investments towards the production of generic biologicals. The production of biosimilars continues to be highly regulated due to its protein nature, unlike other pharmaceutical drugs. The manufacturing equipment is expected to qualify the standards set by regulatory bodies like the Food and Drug Administration (FDA). The unique characteristics of these products require specific functionalities in metering pumps used in this industry. The huge growth of biosimilars across the globe is expected to increase the potential of the market across the globe, from requirements in laboratories to manufacturing.
A Need to Reduce Healthcare Expenditure
As governments struggle to reduce the healthcare expenses of growing populations, the demand for generics is on the rise. Biosimilars, like generic medicines, can substantially reduce healthcare costs. By 2020, eight countries in the European Union (EU) could save a cumulative total of between €11.8 billion ($15.2 billion) and €33.4 billion ($4.38 billion) on their healthcare spending through the use of biosimilar medicines.
The global biosimilars market continues to grow rapidly and is expected to reach a market size of approximately $12.5 billion by 2017. With seven out of the top 10 global drugs being biologicals, major pharmaceutical companies are creating a stronghold for themselves in this market. The emerging biosimilars market has grown significantly in the recent past and has the ability to grow further due to the expiry of pa
tents, government pressures to reduce healthcare costs and the savings that can be obtained over branded drugs.
Safety and Reliability of Metering Pumps offer Growth Potential
The emergence of low-cost biologicals now requires the selection of the proper technology to define its quality in production. A specific expertise is required for the production of drugs not only to meet legal requirements but also to ensure integrity and quality of the end products. In the biotech industry, the specification profiles of pumps are subject to a wide range of international laws, regulations and directive
s. These may include the European Union (EU) Machinery Directives, standards such as DIN EN 12462 Biotechnology, the current Good Manufacturing Practice (cGMP) regulations, U.S. standards such as 3A, and ensuring Food and Drug Administration (FDA) compliance in the use of materials. The greatest possible biological safety must be ensured for the manufacturing process as a whole, and certified clean in place (CIP), for which pumps must be designed without dead spaces or crevices, plays a key part in this. Other important parameters include assured sterilization in place (SIP) or sanitization, and certification concerning the use and origin of materials (3.1 B certificate, FDA compliance).
The safety of production fo
llowed by validation and documentation are the top priorities of this industry. Additionally, the sheer sensitivity of the products compels the industry to pay more attention to the selection of pumps for an application. The regulatory factors and the functional advantage have resulted in an increased demand for peristaltic pumps, which is the highest growth segment for various metering requirements over the past few years. The fluid does not come in contact with the metallic part of the pump, minimizing incidents of contamination. Tubing for the pump is selected based on the nature of the fluid. Other factors include the maintenance of constant pressure and low noise, as well as pulsation levels to ensure the product is not damaged. Hence, minimizing the product loss is the first priority towards optimizing the production process.
Other pumps like diaphragms can also be used for a number of applications in this industry. The 316L stainless steel body and a seal less design minimize the risk of leakage and contamination. These pumps can also handle concentrated toxic fluids as well as fluids with low viscosity. Other positive displacement pumps like the rotary lobe and the progressive cavity pumps also provide sufficient reliability for metering in biopharmaceuticals.
In this highly regulated and versatile industry, it is important for solution providers to not only ensure precise flow control and isolation, but substantiate their capabilities to provide repeated and reliable performance.
Trends in Metering Pumps

Technology and innovation in product development as well as services will play major roles in creating differentiating factors for metering pump vendors. For example, the production landscape in the pharmaceutical industry is seeing a number of changes, such as adoption of continuous or perfusion processing. This would require a pre-defined dosage of chemicals repeated over specific time intervals. These units, as part of a smart pump system, would also support the monitoring of metering pumps from remote locations. The sophisticated levels of the customer’s processes require other services like predictive maintenance, thereby reducing downtime in production. As the future of the manufacturing industry moves towards more intelligent equipment and platforms, solution providers should focus on the development of effective and efficient metering pumps that can cater to the evolving requirements of the industry.
Apart from creating specific products such as valve-less metering pumps, or including integral variable frequency drive (VFD)/motor controls for improved precision metering control, effective value can be provided through product bundling strategies and services. This may include options such as 3-D modeling and design of the equipment to ensure optimum performance and implementation.
Vast opportunities exist in the metering pumps market for suppliers to enhance their growth prospects in special pockets of the pharmaceuticals industry. Smart metering coupled with services and government initiatives such as regulations are likely to fuel the metering pumps market.
For more information, visit www.frost.com or contact Britni Myers at 210-477-8481.